The DICQ (Departamento de Inspeção e Controle de Qualidade), one of the branches of the National Accreditation System, has as its main objective to promote the continuous improvement of quality in clinical laboratories and other areas of health in Brazil. By seeking accreditation, your laboratory demonstrates commitment to:
- Quality: Implementation of standardized and controlled processes, resulting in more accurate and reliable exams.
- Technical Competence: Continuous qualification and training of the team, ensuring excellent results.
- Safety: Safe and efficient work environment, with reduced risks and better process management.
- Reliability: Recognized in the market for its excellence, generating greater security for doctors and patients.
- Credibility: Differentiation in the market through certification that guarantees compliance with quality standards.
How Can Greiner Bio-One's eTrack System Help?
We understand the challenges your laboratory faces in its quest for accreditation, which is why we offer a complete and integrated solution: the eTrack system. Our software was designed to assist in quality management and control, facilitating the achievement of certifications:
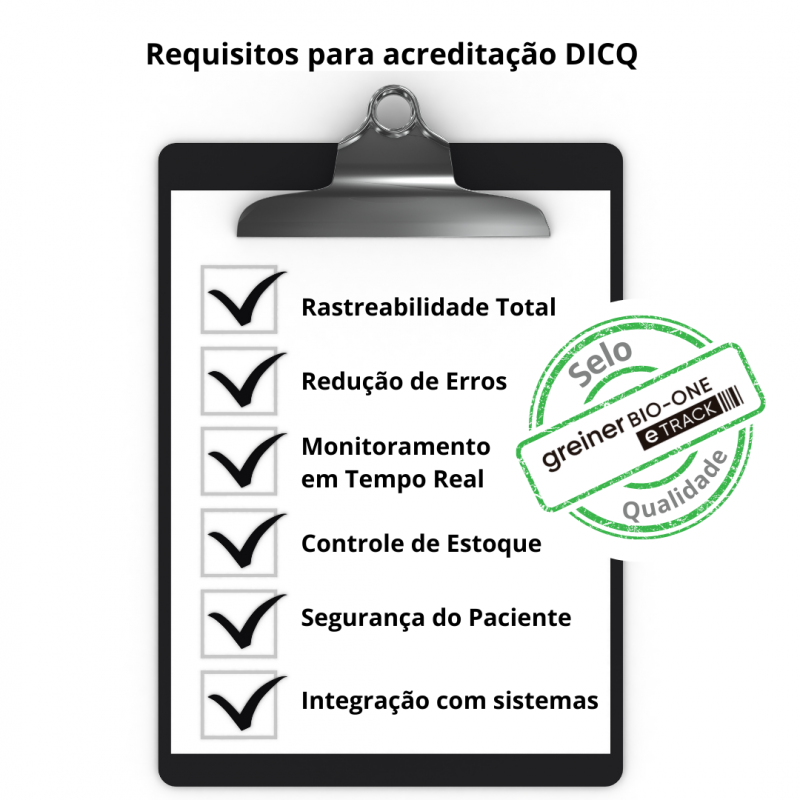
- Full Traceability: With eTrack, each stage of the analytical process is tracked, from collection to report issuance. This ensures compliance with the DICQ requirements on traceability, which is essential for audits.
- Error Reduction: Eliminate the possibility of errors due to handwritten records. eTrack automates processes and validations, preventing errors and increasing efficiency, in addition to ensuring reliable and consistent results.
- Real-Time Monitoring: Monitor all processes in your laboratory, generating information and reports that can be used for decision-making and continuous quality monitoring.
- Inventory Control: Inventory control through traceability and barcode reading allows greater reliability in samples, reagents, disposable materials and validity control, helping with costs and eliminating waste.
- Patient Safety: Eliminate the possibility of sample mix-ups and identification errors.
- Integration with Other Systems: eTrack can be integrated with other laboratory information systems (LIS), optimizing flows and ensuring more efficient management of your laboratory.
eTrack and DICQ: A Perfect Combination for Your Success!
With eTrack, the accreditation process becomes simpler, faster and more efficient. Our system allows you to:
- Organization and standardization of processes: Facilitates the preparation of quality manuals and work instructions.
- Quality Assurance: Provides reliable and detailed information to monitor each process.
- Custom Reports: Allows you to generate comprehensive reports that demonstrate your quality control and process traceability for auditing purposes.
- Compliance with DICQ requirements and current standards: Ensures that you are always one step ahead of accreditation requirements.
- Audit Preparation: Complete process traceability and document standardization assist the laboratory during audits.
The Next Step is Accreditation!
Don’t waste time and take your laboratory to the next level with eTrack from Greiner Bio-One. Get in touch and find out how we can help you with your accreditation process!
Visit our website: gboetrack.com and speak to one of our experts.

